Which of the Following Compounds Has the Lowest Viscosity
B Butane C4H10 is a gas at STP while pentane C5H12 is a liquid. Which of the following liquids has the lowest viscosity.
Which of the following has the lowest viscosity at a given temperature of all.

. Molecule A and B R I summers of each other both ice summers of obtain which is see C seven age 16. By convention an increase in the number of hydrogen bonds of a compound means an increase in the viscosity. SbBr3 AsBr5 SBr2 BeBr2 OBr2.
CH 2F 2 has a dipole moment of 193 D and a boiling point of 52C. The molecule with the MOST hydroxyl groups ethylene glycol has the HIGHEST viscosity. Which of the following compounds has the highest boiling point.
Which of the following liquids would have the lowest viscosity factoring in both the impact of the substance and the temperature. Ethanol has the lowest vapor pressure and strongest intermolecular force due to hydrogen bonding c. A CCL U B N2 g 29.
This is a Most important question of gk exam. Which of the following compounds has the lowest viscosity. Kerosene is a mixture of hydrocarbons.
H2S H2Se AsH3 H2O. Which of the following compounds has the lowest viscosity. C5H12 is less than C6H14 C2H5OH is less than C2H6 C8H18 is less than C2H6.
Glycol has maximum viscosity because it has two O H groups and there is greater intermolecular H bonding in comparison to water and methanol. Which one of the following has a low density. Which of the following compounds has the lowest viscosity.
Water has the lowest viscosity. More dramatically a long-chain hydrocarbon like squalene C 30 H 62 has a viscosity an order of magnitude larger than the shorter n-alkanes. And those different properties including the would include viscosity.
We review their content and use your feedback to keep the quality high. C 5H 11OH at 75C 32. View the full answer.
Water experiences hydrogen bonding so it has a higher surface tension than acetone which only has dipole-dipole interactions. HCl g Expert Answer. The compound HOCH2CH2OH named ethylene glycol relative to the other compounds has more hydrogen bonding.
94 18 ratings a N2 has lowest viscosity because the forces of at. C 3H 7OH at 25C b. Choose the substance with the lowest viscosity.
Solve any question of Mechanical Properties Of Fluids with-. Asked 9302020 50149 PM. Provide an explanation for the following physical properties.
Put the following compounds in order of decreasing viscosity 1 is the highest and 4 is the lowest. Rank the following compounds by surface tension from lowest to highest. We would expect a to be more viscous.
Heptane has lower vapor pressure than acetone due to London dispersion forces. In the molecule of the hydrogen bonding forces tend to resist any phase transition form liquid state to gaseous phase and these hydrogen bonding forces are greater usually when the hydrogen is bound to an electronegative atom like chlorine fluorineoxygenetc. And because they are ice summers they have different structures which is going to give them different properties.
This answer has been confirmed as correct and helpful. A Water beads up on your windshield but acetone doesnt. The liquid which would have the highest viscosity at room temperature is.
Kerosene is a mixture of hydrocarbons. Substances composed of longer molecules tend to have larger viscosities due to the increased contact of molecules across layers of flow. Experts are tested by Chegg as specialists in their subject area.
Substance Viscosity CPMercury 153Water 089Kerosene 164Glycerine 950So glycerine has the greatest viscosity. MgBr 2 at 25C d. Updated 9302020 81603 PM.
D Le Chateliers principle E none of these Which of the following compounds has the lowest viscosity. Well the longest molecule diethyl ether HAS the lowest viscosity. Rank the following compounds by viscosity from most viscous to least.
A surface tension 30. Salt content measured as sodium chloride in electrically desalted crude oil comes down to a level of about __________ ptb pounds per thousand barrel. Which of the following has the lowest viscosity at a given temperature of all.
This then lowers the viscosity of the compound. Log in for more information. Choose the substance with the highest vapor pressure at a given temperature.
Which of the following has the lowest viscosity at a given temperature of. C 5H 11OH at 25C e. C 3H 7OH at 75C c.
D CH3- CH22s-CHs E HCl g What is responsible for capillary action a property of liquids. Up to 256 cash back Get the detailed answer. C5H12 CH4 C3H8 C2H6 C4H10.
Given below are the temperatures at which two different liquid compounds with the same empirical formula have a vapor pressure of 400 torr. CCl4l N2l H2Ol CH3-CH225-CH3l HCll The normal boiling point of dimethyl ether will be higher than the normal boiling point of ethanol. Who are the experts.
This effect can be observed for the n-alkanes and 1-chloroalkanes tabulated below. We would ASSUME that the molecule with the MOST hydroxyl groups has the greatest degree of intermolecular force and this proposition is supported by the data. Sulfur dioxide - molecular because it is made up of all non-metals and has a relatively low MP -187 oc Iron - metal because Iron Fe is a metal.
B cohesive forces C adhesive forces D viscosity E two of these. Ethylene glycol has highest surface tension and. Which of the following pairs correctly ranks the viscosity of the compounds.

Viscosity An Overview Sciencedirect Topics
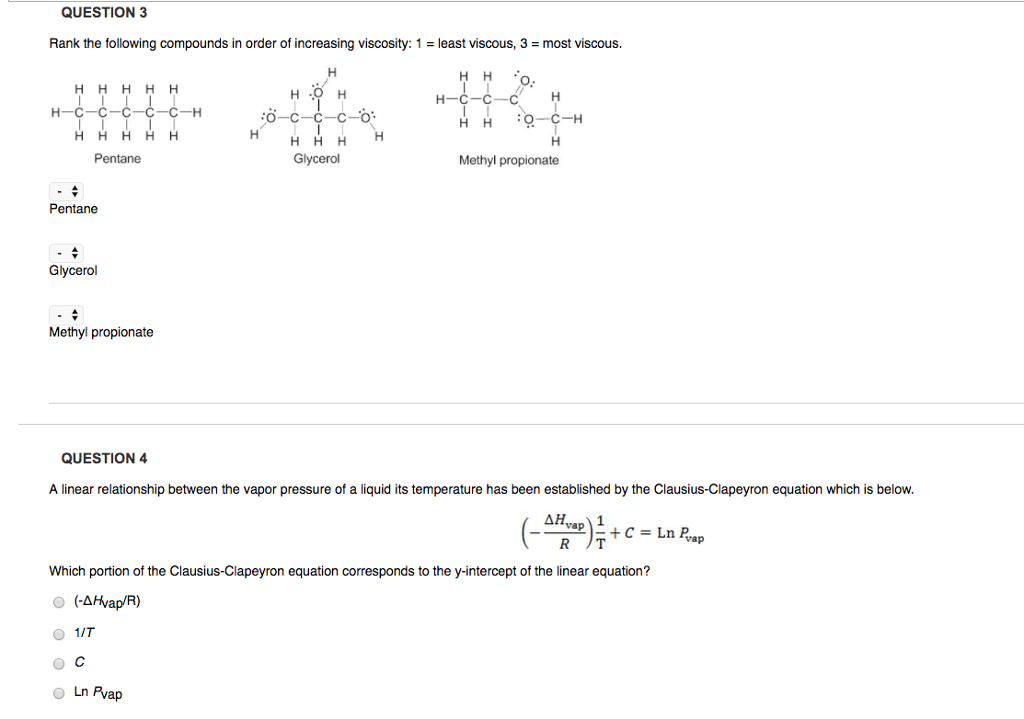
Solved Rank The Following Compounds In Order Of Increasing Chegg Com

Organic Chemistry Science Educational School Posters Organic Chemistry Chemistry Classroom Teaching Chemistry

No comments for "Which of the Following Compounds Has the Lowest Viscosity"
Post a Comment